Mm Hg To Mm H2o
timefordiamonds
Sep 11, 2025 · 5 min read
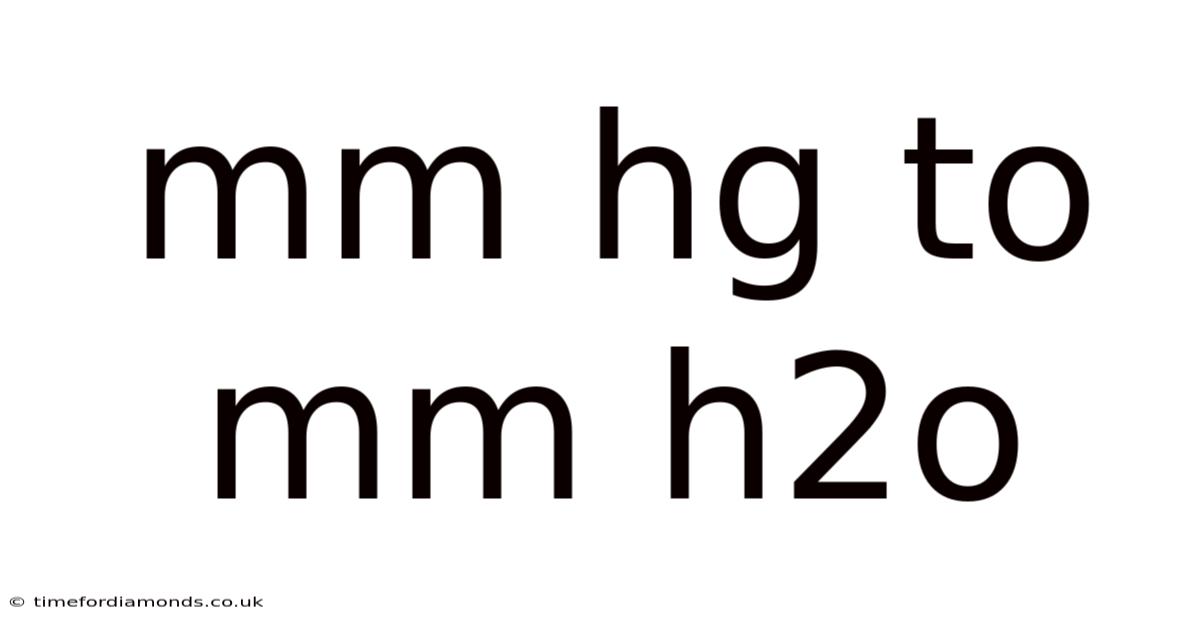
Table of Contents
Converting mmHg to mmHg2O: A Comprehensive Guide
Understanding pressure measurements is crucial in various fields, from medicine and meteorology to engineering and aviation. Two common units used to express pressure are millimeters of mercury (mmHg) and millimeters of water (mmH₂O). While both measure pressure, they differ significantly in their reference liquids – mercury and water, respectively. This article provides a comprehensive guide to converting mmHg to mmH₂O, explaining the underlying principles, the conversion formula, practical applications, and frequently asked questions. This conversion is essential for accurate comparisons and calculations across different pressure measurement systems.
Understanding Pressure and its Units
Pressure is defined as the force exerted per unit area. In simpler terms, it's how much force is pushing on a given surface. The units used to measure pressure reflect this definition and the substance used to create the pressure.
-
Millimeters of Mercury (mmHg): This unit historically stems from the use of a mercury barometer. A mmHg is the pressure exerted by a column of mercury one millimeter high. Mercury is a dense liquid, meaning a relatively short column exerts significant pressure. This unit is commonly used in medical contexts (blood pressure) and some scientific applications.
-
Millimeters of Water (mmH₂O): This unit, similar to mmHg, uses a column of water to define pressure. A mmH₂O is the pressure exerted by a column of water one millimeter high. Since water is less dense than mercury, a taller column of water is needed to exert the same pressure as a shorter column of mercury. This unit finds applications in various fields, particularly those involving less substantial pressures, like measuring the pressure in water pipes or ventilation systems.
The Conversion Formula: mmHg to mmH₂O
The key to converting between mmHg and mmH₂O lies in understanding the density difference between mercury and water. The conversion formula is based on the ratio of their densities:
mmH₂O = mmHg × 13.6
Where:
- 13.6 represents the approximate ratio of the density of mercury to the density of water at standard temperature and pressure (STP). This ratio can vary slightly depending on temperature and purity of the liquids, but 13.6 is a widely accepted approximation for most practical applications.
Step-by-Step Conversion Process
Let's illustrate the conversion process with a few examples:
Example 1: Convert 760 mmHg to mmH₂O.
-
Identify the mmHg value: 760 mmHg
-
Apply the conversion formula: mmH₂O = 760 mmHg × 13.6
-
Calculate the mmH₂O value: mmH₂O = 10336 mmH₂O
Therefore, 760 mmHg is equivalent to 10336 mmH₂O.
Example 2: Convert 120 mmHg to mmH₂O.
-
Identify the mmHg value: 120 mmHg
-
Apply the conversion formula: mmH₂O = 120 mmHg × 13.6
-
Calculate the mmH₂O value: mmH₂O = 1632 mmH₂O
Thus, 120 mmHg is equal to 1632 mmH₂O.
Example 3: Convert 50 mmH₂O to mmHg.
To convert mmH₂O back to mmHg, simply reverse the formula:
mmHg = mmH₂O / 13.6
-
Identify the mmH₂O value: 50 mmH₂O
-
Apply the reversed conversion formula: mmHg = 50 mmH₂O / 13.6
-
Calculate the mmHg value: mmHg ≈ 3.68 mmHg
Scientific Explanation of the Density Ratio
The density ratio of 13.6 between mercury and water is fundamental to the conversion. Density is mass per unit volume. Mercury has a much higher density than water because its atoms are more tightly packed. This means that a smaller volume of mercury possesses the same mass as a larger volume of water. Consequently, a shorter column of mercury can exert the same pressure as a much taller column of water. This density difference is the reason for the significant numerical difference in the two pressure units when representing the same pressure. The accurate determination of this density ratio requires precise measurements under controlled conditions.
Practical Applications of mmHg to mmH₂O Conversion
The ability to convert between mmHg and mmH₂O is vital in various fields:
-
Medicine: While blood pressure is typically measured in mmHg, understanding the equivalent mmH₂O value can be helpful for comparing pressures in different physiological contexts or for calculations involving fluid dynamics in the circulatory system.
-
Environmental Science: In studying atmospheric pressure or water column pressures in hydrological studies, converting between units ensures consistent data analysis and comparison.
-
Engineering: In designing systems involving fluid flow and pressure regulation (e.g., water supply systems, ventilation systems), converting between mmHg and mmH₂O helps engineers choose appropriate components and ensure the systems operate within safe and efficient parameters.
-
Industrial Processes: Many industrial processes involve pressure control and monitoring. The ability to convert between units ensures seamless integration of different measuring devices and control systems.
Frequently Asked Questions (FAQ)
Q1: Is the density ratio of 13.6 always accurate?
A1: The density ratio of 13.6 is an approximation. The actual ratio can vary slightly depending on temperature and the purity of the mercury and water. However, for most practical purposes, this approximation is sufficiently accurate.
Q2: Can I use this conversion for other liquids besides mercury and water?
A2: No, this specific conversion formula is only applicable to mmHg and mmH₂O. To convert pressures involving other liquids, you would need to use the appropriate density ratio for those liquids.
Q3: What are some common sources of error in pressure measurements?
A3: Errors can arise from various sources, including inaccuracies in measuring instruments, temperature fluctuations affecting liquid densities, and variations in gravitational acceleration.
Q4: Are there other units used to measure pressure?
A4: Yes, many other units exist, including Pascals (Pa), atmospheres (atm), bars, pounds per square inch (psi), and inches of mercury (inHg). Conversion factors exist to relate these units to mmHg and mmH₂O.
Conclusion
Converting between mmHg and mmH₂O is a fundamental calculation in various scientific and engineering disciplines. Understanding the underlying principles, particularly the density difference between mercury and water, is crucial for performing accurate conversions. By applying the simple formula (mmH₂O = mmHg × 13.6), you can readily transform pressure values between these two common units, ensuring consistent and reliable data analysis across different applications. Remember that while 13.6 is a good approximation, slight variations may occur due to temperature and purity differences. Always strive for accuracy in your measurements and calculations to ensure the reliability of your results. The ability to perform this conversion enhances your understanding of pressure measurement and expands your capacity to work effectively across diverse scientific and engineering fields.
Latest Posts
Latest Posts
-
Convert 70 F To Celsius
Sep 11, 2025
-
900 Square Feet In Meters
Sep 11, 2025
-
Convert 33 Kg To Pounds
Sep 11, 2025
-
Convert 33 Kg To Lbs
Sep 11, 2025
-
Kilos En Libras Cuanto Es
Sep 11, 2025
Related Post
Thank you for visiting our website which covers about Mm Hg To Mm H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.